Ready to Use Sterile Vials USA
USP-compliant pharmaceutical glass vials — sterile, contamination-free, and ready for immediate filling and use
Ready to Use (RTU) Sterile Packaging Solutions
(Pre-Washed, Pre-Sterilized, Depyrogenated Vials)
At Livealth Biopharma, we specialize in manufacturing and supplying high-quality Ready to Use (RTU) vials and cartridges, engineered to meet the exacting standards of the US pharmaceutical and healthcare industries. Backed by GMP-certified facilities and advanced automation, our solutions are designed to deliver reliability, precision, and uncompromising safety.
We understand that every pharmaceutical product demands the highest levels of sterility and compliance. That’s why our RTU range is pre-washed, depyrogenated, and pre-sterilized, ensuring seamless integration into aseptic filling lines and reducing the need for in-house washing or sterilization steps. This commitment to excellence helps our partners bring critical therapies to market faster, while maintaining regulatory compliance.
Why Choose Livealth Biopharma as Your Trusted RTU Pharma Supplier in India?
Affordable Generic Drugs
All Injectable Manufacturer under one roof
Dealing in Orphan Products

Dealing in flexible MOQ as per client requirement

Dealing in both Human and Veterinary division

Dealing in Finished Products and API's
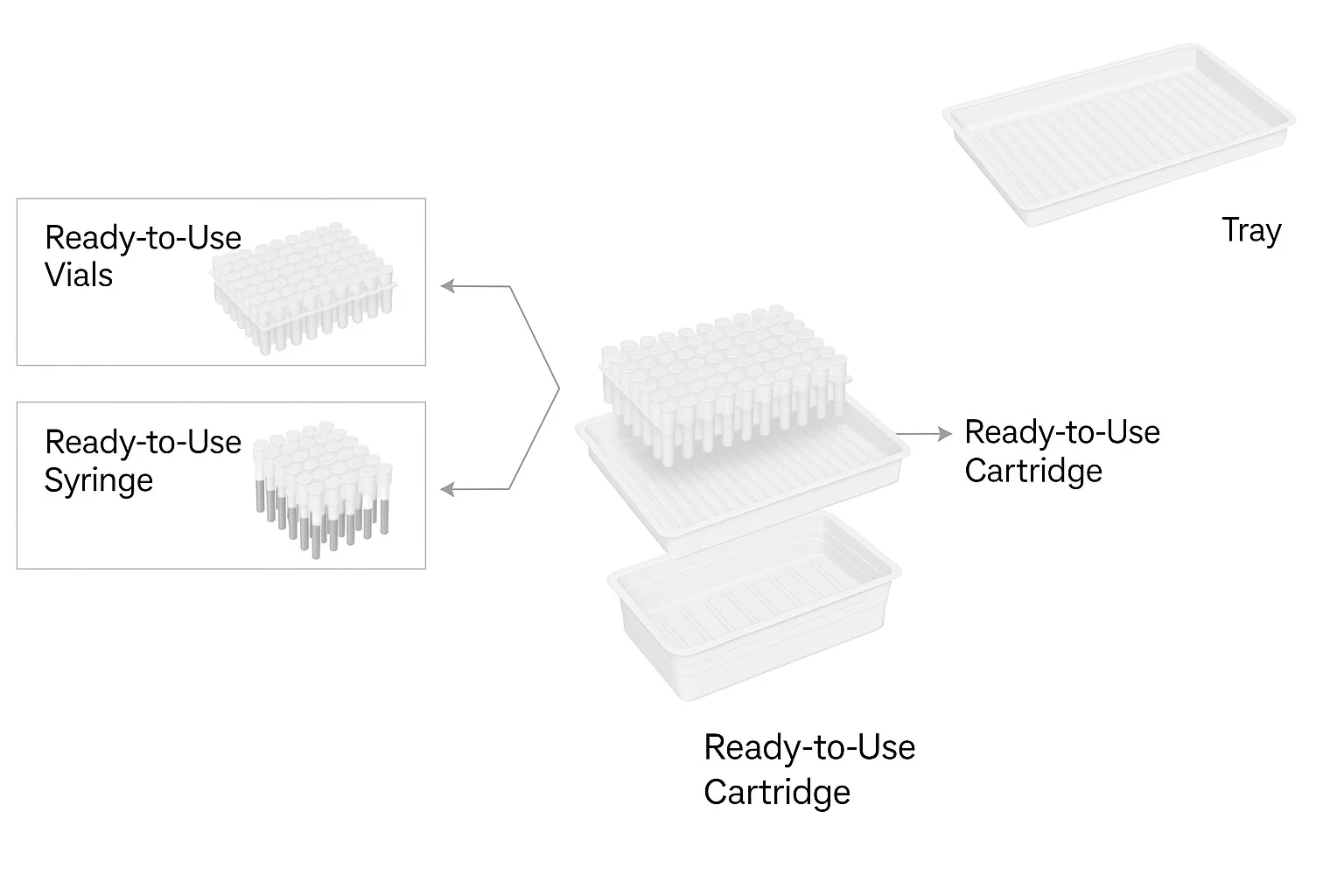
OUR RTU PRODUCT PORTFOLIO
Technical Details & Compliance for RTU Vials with Nest and Tub
Our Ready to Use (RTU) vials with nest and tub configurations are crafted from high-quality materials and comply with internationally recognized standards. Designed to ensure sterility, safety, and performance, these vials are ideal for ready-to-fill operations serving regulated markets including the USA and EU.
Sterilization methodETO (Ethylene Oxide)
| Components | Material Description |
|---|---|
| Glass injection vials | Borosilicate glass |
| Nest | Polypropylene (PP) |
| Tub | High-impact polystyrene (HIPS) |
**All components and processes are validated under strict GMP-certified environments to deliver the highest level of sterility and compliance required by pharmaceutical manufacturers worldwide.

Download Our Detailed RTU Vials & Cartridges Brochure
Explore full specifications, material compliance, sterilization methods, and packaging solutions in our comprehensive brochure.
Technical Details & Compliance for RTU Vials with Tray
| Components | Material Description |
|---|---|
| Glass injection vials | Borosilicate glass |
| Tray | Polypropylene (PP) |
| Sterilization method | ETO (EO) |
Download Our Detailed RTU Vials & Cartridges Brochure
Explore full specifications, material compliance, sterilization methods, and packaging solutions in our comprehensive brochure.
Product Assemblies – RTU Cartridges
| Components | Material Description |
|---|---|
| Cartridge barrels | Borosilicate glass |
| Rubber stopper | Bromobutyl rubber, Chlorobutyl rubber |
| Alu-cap seal with combiseal | Aluminum cap, Combiseal: Bromobutyl rubber, Polyisoprene rubber |
| Silicone oil | DC360 |
| Nest | PP |
| Tub | PS |
| Sterilization method | ETO |
Product Specifications
| Specifications | d1 Nominal | d1 Tolerance | d2 Nominal | d3 Min | d4 Nominal | d5 Nominal | d6 Nominal | h1 Nominal | h1 Tolerance | h2 Nominal | h2 Tolerance |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1ml | 8.65 | ±0.1 | 6.85 | 6.55 | 7.15 | 5.5 | 3.15 | 5.0 | ±0.2 | 2.9 | ±0.1 |
| 1.5ml | 8.65 | ±0.1 | 6.85 | 6.55 | 7.15 | 5.5 | 3.15 | 5.0 | ±0.2 | 2.9 | ±0.1 |
| 1.8ml | 8.65 | ±0.1 | 6.85 | 6.55 | 7.15 | 5.5 | 3.15 | 5.0 | ±0.2 | 2.9 | ±0.1 |
| 3ml | 11.60 | ±0.1 | 9.65 | 9.35 | – | – | – | – | – | – | – |
**All dimensions in millimeters; according to ISO13926-1:2018
Download Our Detailed RTU Vials & Cartridges Brochure
Explore full specifications, material compliance, sterilization methods, and packaging solutions in our comprehensive brochure.
Have questions about packaging? Contact us today.

Advantages of Livealth RTU Vials Solutions
-
Ultra-Clean Intelligent Production: Automated production in GMP-certified cleanrooms (Class C + Class A) with AI-based inspection and micron-level precision.
-
Enhanced Product Quality: Superior mechanical performance and appearance; fewer process steps reduce downtime and risk.
-
Cost-Efficient Mode: Eliminates need for costly washing, depyrogenation, and tunnel ovens; reduces capital expenditure and factory footprint.
Advantages of Our Ready to Use Sterile Vials
At Livealth Biopharma, we bring the highest manufacturing standards to our Ready to Use Sterile Vials, trusted by pharmaceutical and biotech companies across the United States:
-
Ultra-Clean Intelligent Production
Manufactured in GMP-certified cleanrooms (Class C + Class A) with 100% visual and AI inspection to ensure precise sealing and safety for even the most sensitive drugs. -
Enhanced Product Quality
Sterile packaging delivers better appearance, higher mechanical strength, and fewer processing steps—helping reduce downtime and operational risks. -
Cost-Efficient Mode
Designed for “Ready-to-Fill” use straight out of the box, eliminating the need for washing machines, water systems, and tunnel ovens. This cuts capital expenses, saves space, and lowers total production costs by up to 80%.
Applications
Our Ready to Use Sterile Vials support a wide range of pharmaceutical and biotech needs in the US, including:
-
Generics and chemicals
-
Biologics
-
Low-filled and high-value drugs
-
New drug development
These versatile applications make them ideal for CDMOs, hospitals, compounding pharmacies, and research institutions across the United States.
Storage & Transport Recommendations
To maintain the integrity and sterility of our Ready to Use Sterile Vials, we recommend:
-
Packaging Protection
Using secure, sealed packaging to prevent contamination and protect against damage during shipping. -
Temperature and Humidity Control
Avoid exposure to high temperatures and humidity that can lead to moisture absorption, mold growth, or bacterial contamination. -
Vibration and Shock Protection
Minimize pressure, vibration, and shocks during transport to preserve product quality and sterility. Products should also be stored away from hazardous or contaminating substances.
FAQs on Ready to Use Sterile Vials
What are Ready to Use (RTU) vials?
Ready to Use (RTU) vials are pre-washed, depyrogenated, and terminally sterilized glass vials designed for immediate filling, saving time and reducing contamination risks in pharmaceutical production.
Do you offer pharmaceutical glass vials for the USA market?
Yes. At Livealth Bio Pharma, we specialize in supplying pharmaceutical glass vials and sterile vials exclusively to clients across the USA.
What sizes of sterile glass vials do you manufacture?
We offer a range of glass vials for pharmaceutical use, including various neck finishes and capacities, to meet your specific sterile filling requirements.
Why choose RTU vials instead of standard glass vials?
RTU vials reduce in-house processing steps like washing and depyrogenation, lower equipment costs, and improve operational efficiency, making them ideal for sterile drug manufacturing.
Are your ready to use vials compliant with US regulations?
Absolutely. Our sterile glass vials meet stringent US FDA, USP, and ISO standards to ensure safety, sterility, and consistent quality.
Do you ship sterile vials internationally?
Currently, we only supply RTU sterile vials and pharmaceutical glass vials within the USA.
How can I get a quote for RTU vials?
Simply fill out our online form, and our sales team will promptly share product details, pricing, and lead times tailored to your needs.
Who should use ready to use sterile glass vials?
These vials are ideal for pharmaceutical manufacturers, biotech companies, and contract manufacturing organizations that require sterile vials ready for aseptic filling.
What RTU vial formats do you offer?
We supply a comprehensive RTU product portfolio, including:
-
RTU vials with Nest & Tub for automated filling lines
-
RTU vials with Tray for flexible batch sizes
-
RTU cartridges for injectable and specialty products